| Cellular and Molecular Medicine Research, ISSN 2817-6359 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cell Mol Med Res and Elmer Press Inc |
| Journal website https://www.thecmmr.org |
Original Article
Volume 1, Number 1, September 2023, pages 20-26
Formononetin: A Phytoestrogen and Isoflavone, Relaxes Guinea Pig Gallbladder Strips
Loren W. Klinea, c, Edward Karpinskib
aDepartment of Dentistry, University of Alberta, Edmonton, AB T6G2E1, Canada
bDepartment of Physiology, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB T6G2H7, Canada
cCorresponding Author: Loren W. Kline, Department of Dentistry, University of Alberta, Edmonton, AB T6G 2E1, Canada
Manuscript submitted May 26, 2023, accepted July 30, 2023, published online August 30, 2023
Short title: Formononetin and Gallbladder Motility
doi: https://doi.org/10.14740/cmmr16e
| Abstract | ▴Top |
Background: Formononetin, a phytoestrogen and isoflavone, is present in red clover and several types of beans. It relaxes vascular smooth muscle and prostate smooth muscle. The purpose of this study was to determine if formononetin had an effect of gallbladder motility.
Methods: An in vitro technique was used to determine which system mediated the relaxation. Formononetin (FMN) relaxed cholecystokinin octapeptide- (CCK) and KCl-induced tension in male guinea pig gallbladder strips in a concentration dependent manner. Both paired t-tests and analysis of variance were used for statistical analysis; differences between mean values of P < 0.05 were considered significant.
Results: Adding FMN prior to CCK or KCl produced a significant decrease in the amount of tension (1.2 ± 0.07 g vs. 0.81 ± 0.05 g CCK, P < 0.001; 0.84 ± 0.04 g vs. 0.74 ± 0.05 g KCl, P < 0.01). When the protein kinase C (PKC) inhibitors bisindolymaleimide IV and chelerythrine Cl- were used together, a significant (P < 0.01) reduction in the FMN-induced relaxation (78.1±4.5% vs. 69.7±3.4%) was observed. Genistein, a protein tyrosine kinase inhibitor, significantly (P < 0.01) decreased the amount of FMN-induced relaxation (72.2±6.5% vs. 59.3±6.5%). To determine if protein kinase A (PKA) mediated the FMN-induced relaxation, PKA inhibitor 14-22 amide myristolated (PKA-IM) was used. PKA-IM had no significant effect on the amount of FMN-induced relaxation. Neither the use of 2-aminoethoxydiphenyl borate (2-APB), a blocker of intracellular Ca2+ release; KT5823, a blocker of protein kinase G; or L-NG-methyl-L-arginine acetate salt (L-NMMA), a nitric oxide synthase inhibitor, had a significant effect on the amount of FMN-induced relaxation.
Conclusions: The FMN-induced relaxation of CCK- or KCl-induced tension was mediated by blocking extracellular Ca2+ entry which then affected downstream events such as activation of PKC and protein tyrosine kinase.
Keywords: Formononetin; Phytoestrogen; Isoflavone; Gallbladder; Smooth muscle
| Introduction | ▴Top |
Medicinal plants, such as turmeric and ginger, have been used as traditional remedies for thousands of years. Astragalus mongholicus bunge is a medicinal herb that has been used for the treatment of inflammatory diseases [1], nephritis [2], and cardiovascular diseases [3]. The phytoestrogen, formononetin (FMN) [7-hydroxy-3-(4-methoxyphenyl) chromen-4-one], is present in Astragalus mongholicus, as well as, Trifolium pratense L. (red clover) and several varieties of beans [4, 5]. Phytoestrogens are classified into three categories: isoflavones, coumestans, and lignans. FMN is an isoflavone [6]. FMN inhibits both growth and MAP kinase activity in human aortic smooth muscle cells. FMN may then confer protective effects on the cardiovascular system by inhibiting vascular remodeling and neointima formation, and may be clinically useful as a safer substitute for feminizing estrogens [7]. FMN relaxed rat isolated aorta through endothelium-dependent and endothelium-independent pathways. The FMN-induced vasodilatation was mediated by nitric oxide (NO) and the activation of Ca2+-activated K+ channels (BKCa) and ATP dependent K+ channels (KATP) [8]. Zhao et al [9] found that FMN had a vasorelaxant effect on the rat thoracic aorta which was mediated by NO, blockade of Ca2+ channels, and the opening of K+ channels. Sun et al [10] reported that FMN upregulated NO synthase in arterial endothelium through estrogen receptors, ERK1/2, and JNK pathways. FMN affects the motility of vascular smooth muscle. Phytoestrogens have been proposed as an alternative to estrogen replacement therapy [11]. Since there are no reports of FMN having an effect on gastrointestinal smooth muscle and phytoestrogens may have an increasing role as estrogen replacements, the purpose of this paper was to determine if FMN had an effect on guinea pig gallbladder smooth muscle motility and the mechanism which mediated its effect.
| Materials and Methods | ▴Top |
Tissue preparation
The experiments were performed under a protocol (#275) re-approved (January 11, 2016 and February 3, 2017) by the Animal Care Committee-Health Sciences of the University of Alberta. Male Hartley guinea pigs (225 - 350 g body weight) were killed by decapitation. The gallbladder was removed from the guinea pig. Liver, fat and connective tissue were removed from the gallbladder, and the gallbladder was placed in Krebs-Henseleit solution (KHS) that was gassed with 95% O2 and 5% CO2. The composition of the KHS was (in mM) NaCl, 115; KCl, 5; CaCl2, 2.1; MgSO4, 1.2; NaH2PO4, 1.2; NaHCO3, 25; and glucose, 11. Each gallbladder was cut into strips (1.5 × 0.5 cm) and maintained in Sawyer-Bartlestone chambers filled with KHS, maintained at 37 °C, and gassed with 95% O2 and 5% CO2. An optimum resting tension of 0.7 g was determined previously and used in the study [12-16].
The force developed by the gallbladder strips was measured with Grass FT03 force displacement transducers (Grass Instruments Co., Quincy MA, USA) and recorded on a Grass 7D polygraph (Grass Instruments Co., Quincy, MA, USA). Isolated strips were equilibrated in the chambers for 45 min prior to determining their suitability for use. Each chamber had 2 M (final concentration) atropine, which was added in every experiment, 3 min prior to the addition of 1.0 nM cholecystokinin octapeptide (CCK). The tension was measured. This was followed by three changes of KHS. The test was repeated twice with 25 min between tests. A repeatable minimum active tension of 0.5 g had to be generated by the strips before use. All agents used were added directly to the chambers. All concentrations are reported as the final concentration in the chambers.
Several series of experiments were performed to examine the effects of FMN on tension generated by the gallbladder strips. CCK (1nM) was found to produce a stable long lasting tension after 3 min. This steady tension lasted at least 10 min [12, 17]. In order to determine if FMN could relax CCK- or KCl-induced tension, concentration response curves were generated. The CCK-induced tension was allowed to reach a steady level (3 min). The strips were exposed to one concentration of FMN, the response was observed until the relaxation reached a steady level (approximately 5 min), the KHS was changed for three times, and the strips were allowed to recover for 30 min before testing a different concentration of FMN. The concentration of FMN (15 M) was selected for use in subsequent experiments as it produced a reproducible relaxation. The same procedure was followed to generate a concentration response curve using 40 mM KCl instead of 1 nM CCK. KCl directly depolarizes smooth muscle, and its use is a standard procedure.
In order to determine if the Ca2+ released from the endoplasmic reticulum mediated the FMN-induced relaxation, 2-aminoethoxydiphenyl borate (2-APB), a cell permeable inhibitor of IP3-induced Ca2+ release, was added to the chambers 10 min prior to the CCK. The CCK was then added to the chambers. When the tension reached a steady level, 15 M FMN was added to the chambers. The amount of relaxation was observed. The amount of relaxation observed when FMN only was used was then compared to the amount of relaxation observed when the strips were treated with 2-APB and FMN. This procedure was followed with each agent used.
When the protein kinase A (PKA) inhibitor 14-22 amide myristolated (PKA-IM; 180 nM) was used, it was added to the chambers 15 min prior to CCK to ensure adequate time for entry into the smooth muscle. The use of KT5823 (585 nM), a protein kinase G (PKG) blocker, was added to the chambers 5 min prior to the addition of CCK. Genistein (10 M), a protein tyrosine kinase inhibitor, was added to the chambers 5 min prior to the addition of CCK.
The protein kinase C (PKC) inhibitors, chelerythrine Cl- (5 M) and bisindolymaleimide IV (BIM, 0.5 M), were used together to determine the effects of PKC on FMN-induced relaxation of CCK-induced tension. BIM blocks the translocation of PKC to its site of action while chelerythrine Cl- acts on the catalytic domain of PKC. It was shown previously that using BIM and chelerythrine Cl- in combination produced the most consistent results [12, 15]. They were added to the chambers 5 min prior to the CCK. L-NG-methyl-L-arginine acetate salt (L-NMMA; 20 M), a nitric oxide synthase inhibitor, was used to determine if nitric oxide (NO) mediated the FMN-induced relaxation.
In order to determine if FMN inhibited extracellular Ca2+ entry, 40 mM KCl was used to induce tension in the strips. After the amount of tension generated by the 40 mM KCl was recorded, the KHS was changed three times and the strips allowed to equilibrate for 25 min. FMN (15 M) was then added to the chambers 3 min prior to the addition of 40 mM KCl. The amount of tension generated was recorded and compared to that observed when the KCl was added to the chambers with no FMN.
Tetraethylammonium chloride (TEA, 100 M) was used to determine if the effects of FMN were mediated by inhibiting K+ channels. TEA was added to the chambers 3 min prior to the CCK.
In order to determine if the effects of estradiol (E2) and FMN on gallbladder motility were additive, male guinea pig gallbladder strips were exposed to E2 (50 M). The concentration of E2 used was found previously to induce a reproducible amount of relaxation of CCK-induced tension [16]. The amount of relaxation of 1.0 nM CCK-induced tension was recorded. After a 25-min recovery period, the strips were administered with E2 (50 M) and FMN (15 M) as close to the same time as possible. The amount of relaxation of CCK-induced tension was recorded. The procedure was repeated using FMN (15 M) initially and then the combination of FMN and E2. This procedure determined that the order of E2 or FMN had no effect on the observed responses.
Materials
The following agents were purchased from Sigma Aldrich (St. Louis, MO, USA): CCK, atropine, L-NMMA, 17 estradiol, and TEA. The following agents were purchased from EMD Millipore (Etobicoke, Ontario, Canada): PKA-IM, KT5823, genistein, and 2-APB. FMN, chelerythrine Cl- , and bisindolymaleimide IV were purchased from Cayman Chemicals (Ann Arbor, MI, USA). All agents were dissolved in either distilled water or dimethyl sulfoxide (DMSO). The amount of DMSO (10 L) added to the chambers was determined to have no effect on the strips.
Statistical analysis
Statistical comparisons were done using either t-test, paired t-test, or analysis of variance. Results were expressed as mean ± standard error (SE). Differences among mean values with P < 0.05 were considered significant. The number of gallbladders (animals) used in each experiment were indicated by “n”. Each gallbladder was used to prepare up to four strips; therefore, an “n” of 4 represented the use of up to 16 strips.
| Results | ▴Top |
Concentration-dependent relaxation
CCK was used to generate tension and FMN was added to the chambers to determine if FMN induced a relaxation of the CCK-induced tension (Fig 1a). FMN produced a concentration-dependent relaxation of CCK-induced tension (Fig. 1b). FMN also produced a concentration-dependent relaxation of KCl-induced tension (Fig. 1b). The amount of FMN-induced relaxation using 5.0 M FMN was significantly (38.1±3.1% vs. 20.1±1.7%, n = 8, P < 0.001) greater when CCK was used rather than KCl. The amount of FMN-induced relaxation using 10.0 M FMN was significantly (40.4±2.5% vs. 30.9±2.6%, n = 10, P < 0.001) greater in the CCK-induced tension than the KCl-induced tension. Lastly, the amount of FMN-induced relaxation using 20.0 M FMN was significantly (68.3±4.9% vs. 46.4±5.2%, n = 8) greater in the CCK-induced tension than the KCl-induced tension (Fig. 1b). There were no significant differences in the amount of tension generated by CCK or KCl.
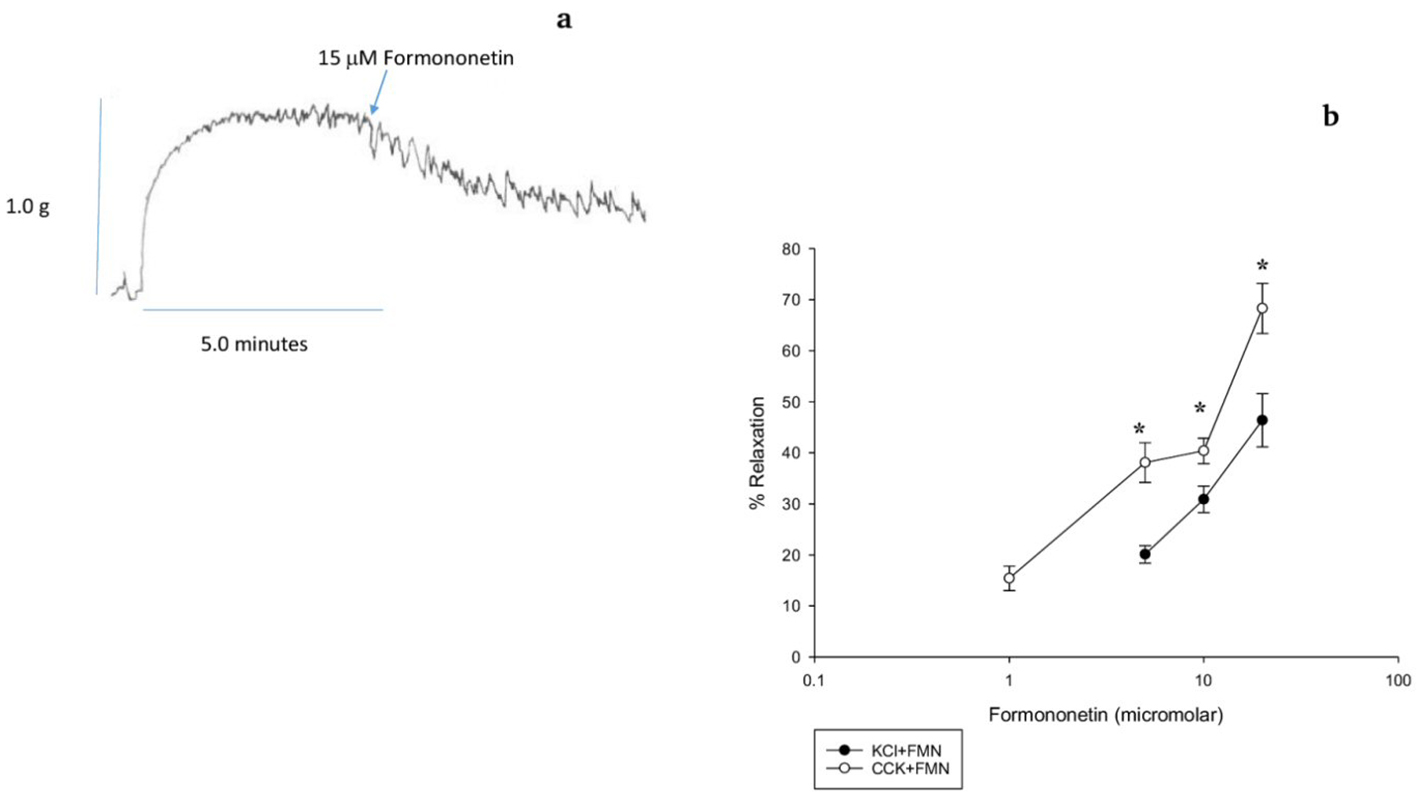 Click for large image | Figure 1. The effect of formononetin (FMN) on CCK-induced tension in a male guinea pig gallbladder strip. (a) Data trace showing the relaxation caused by FMN on CCK-induced tension. (b) FMN (15 M)-induced relaxation of both CCK- (open circles, P < 0.001, n = 8) and KCl- (filled circles, P < 0.001, n = 10) induced tension. Values are means ± SE. FMN (5, 10 and 20 M) significantly (P < 0.001) relaxed CCK-induced tension more than KCl-induced tension. |
Effect of blocking agents
The use of 2-APB, an inhibitor of IP3-induced Ca2+ release, had a significant effect on the amount of CCK-induced tension (0.98 ± 0.06 g vs. 0.37 ± 0.04 g, P < 0.001, n = 5), but had no significant effect on the amount of FMN-induced relaxation (62.2±5.6% vs. 68.2±11.7%, n = 5). The use of the PKC inhibitors BIM and chelerythrine Cl- had no significant effect on the amount of CCK-induced tension with or without FMN (0.86 ± 0.06 g vs. 0.83 ± 0.04 g; n = 5). There was a significant decrease in the amount of FMN-induced relaxation observed between the strips not treated with BIM and chelerythrine Cl- when compared to those treated with the PKC inhibitors (78.1±4.5% vs. 69.7±3.4%; n = 5; P < 0.02; Fig. 2).
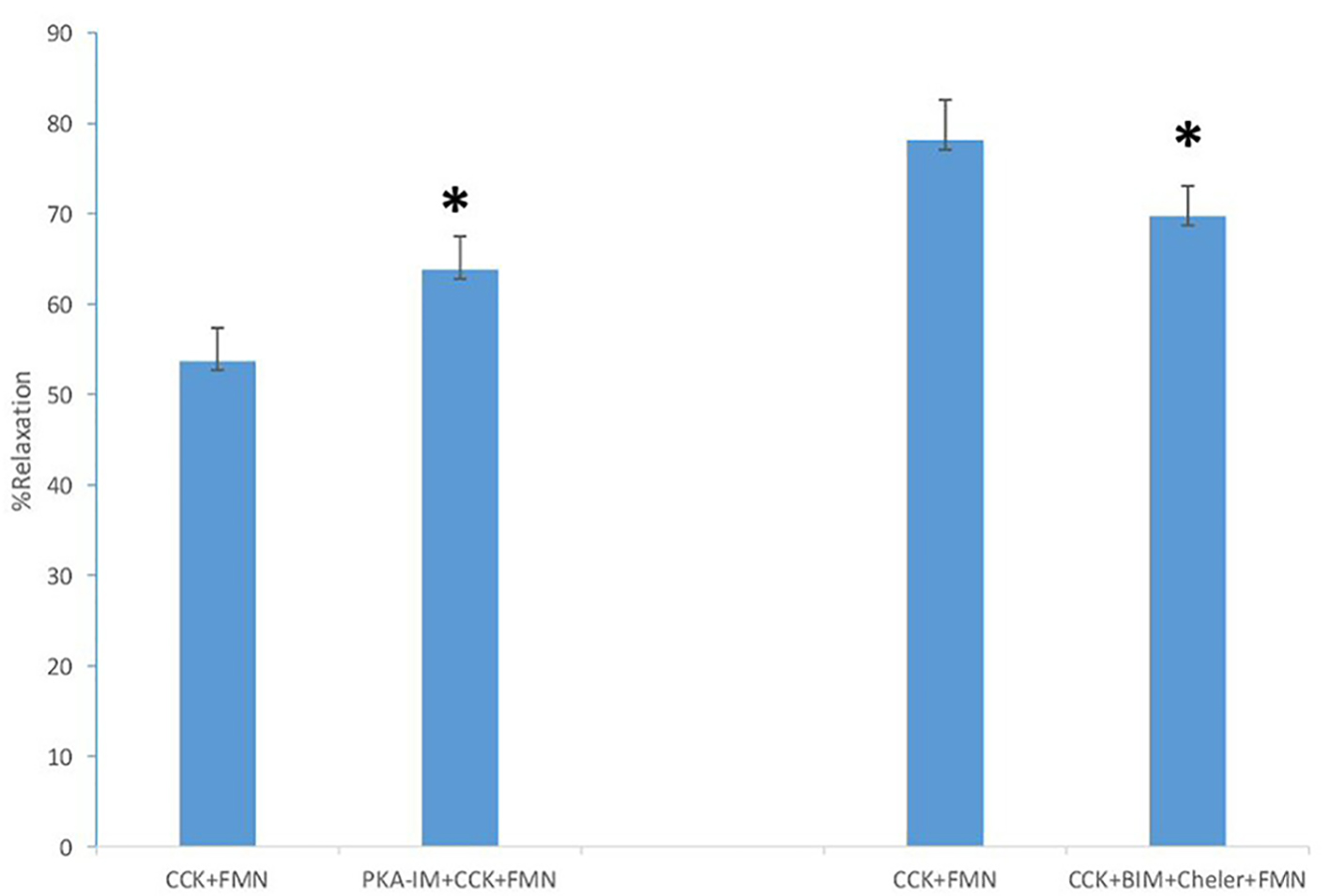 Click for large image | Figure 2. The effect of PKA-IM (180 nM) on FMN-induced relaxation. A significant increase (P < 0.001, n = 5) in the amount of FMN-induced relaxation occurred. The effect of the PKC inhibitors BIM (0.5 M) and chelerythrine Cl- (5 M) on the amount of FMN-induced relaxation (P < 0.02, n = 5). A significant decrease in the amount of FMN-induced relaxation occurred. Values are means ± SE. |
When the PKA inhibitor PKA-IM was used, there was a significant (P < 0.001) increase in the amount of CCK-induced tension (0.90 ± 0.06 g vs. 0.99 ± 0.08 g, n = 5). There was also a significant (P < 0.001) increase in the amount of FMN-induced relaxation (53.5±3.7% vs. 63.8±3.7%, n = 5).
Treatment of the strips with KT5823, a PKG blocker, produced a significant (P < 0.01) increase in the amount of CCK-induced tension (0.75 ± 0.09 g vs. 0.88 ± 0.1 g, n = 4). KT5823 had no significant effect on the amount of FMN-induced relaxation of CCK-induced relaxation (71.6±7.3% vs. 64.4±6.5%, n = 4). Genistein significantly (P < 0.01) decreased the amount of FMN-induced relaxation of CCK-induced tension (72.2±6.5% vs. 59.3±6.5%, n = 6; Fig. 3). There was no significant change in the amount of CCK-induced tension (0.69 ± 0.04 g vs. 0.68 ± 0.05 g). The use of L-NMMA, a nitric oxide (NO) synthase blocker, had no significant effect on the amount of CCK-induced tension (0.90 ± 0.06 g vs. 0.93 ± 0.06 g, n = 5) nor on the amount of FMN-induced relaxation (58.4±3.9% vs. 58.6±4.2%, n = 5).
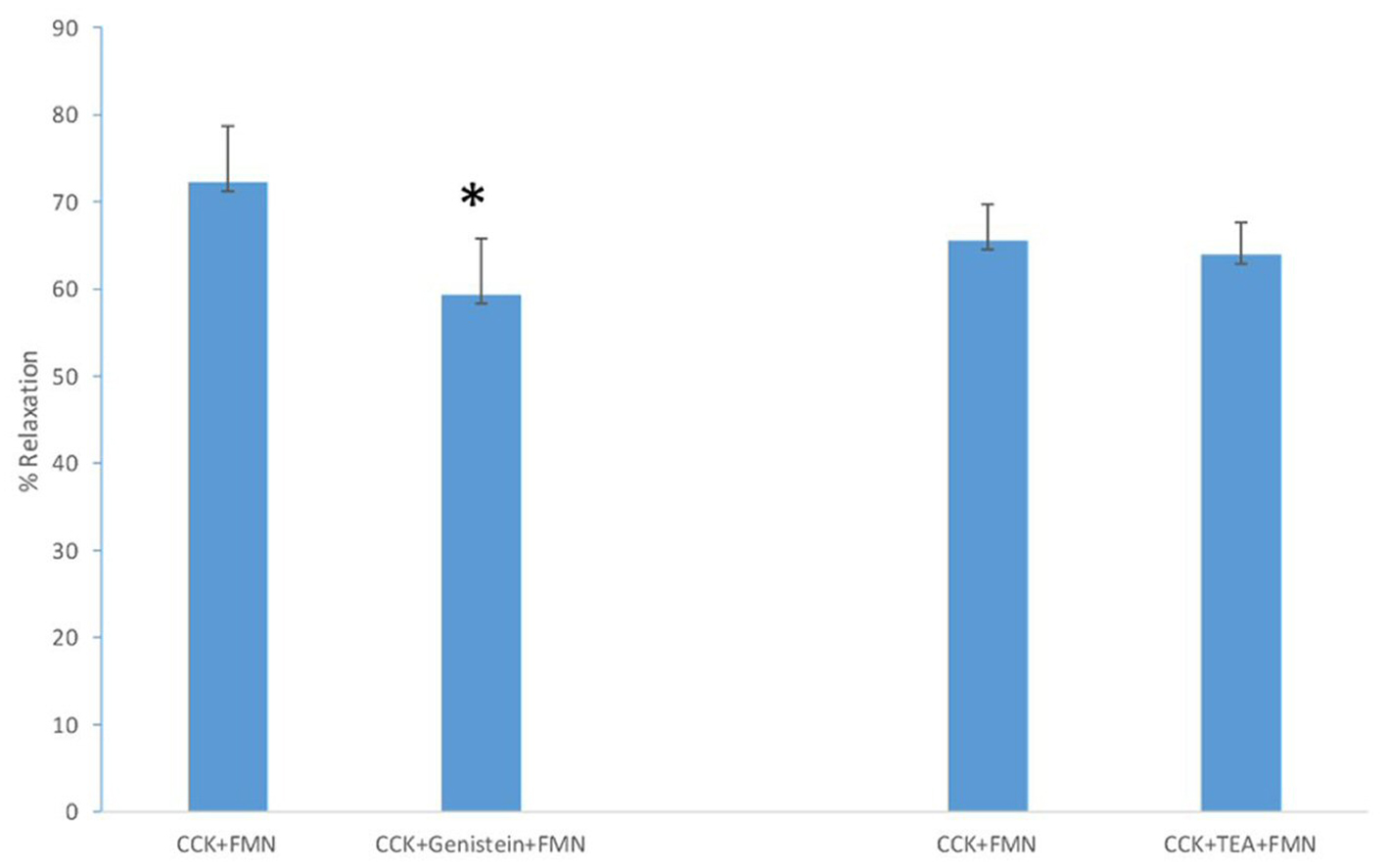 Click for large image | Figure 3. The effect of genistein (10 M, n = 6) on FMN-induced relaxation. A significant (P < 0.01) decrease in relaxation occurred. TEA (50 M, n = 8) had no significant effect on the amount of FMN-induced relaxation. |
When TEA (100 M) was added to the chambers prior to the CCK, a significant (P < 0.01; 0.88 ± 0.04 g vs. 0.97 ± 0.04 g; n = 8) increase in the amount of CCK-induced tension was observed. TEA had no significant effect on the amount of FMN-induced relaxation (65.5±4.2% vs. 63.9±3.7%; n = 8). Fulvestrant, an estrogen receptor blocker, caused a significant (P < 0.01) increase in the amount of CCK-induced tension (0.71 ± 0.05 g vs. 0.81 ± 0.05 g; n = 5), but no significant effect on the amount of FMN-induced relaxation (51.6±5.8% vs. 57.2±7.2%; n = 5).
When FMN was added to the chambers 3 min prior to the addition of KCl (40 mM) there was a significant decrease (P < 0.001) in the amount of tension generated (1.2 ± 0.07 g vs. 0.81 ± 0.05 g, n = 9; Fig. 4). When 15 M FMN was added to the chambers 3 min prior to the addition of CCK (1 nM), there was a significant (P < 0.01) decrease in the amount of tension generated (0.81 ± 0.04 g vs. 0.74 ± 0.05 g, n = 7; Fig. 4). When the amount of the FMN-induced decrease in tension induced by CCK and KCl were compared, FMN significantly (P < 0.001) decreased the amount of KCl-induced more than the CCK-induced tension.
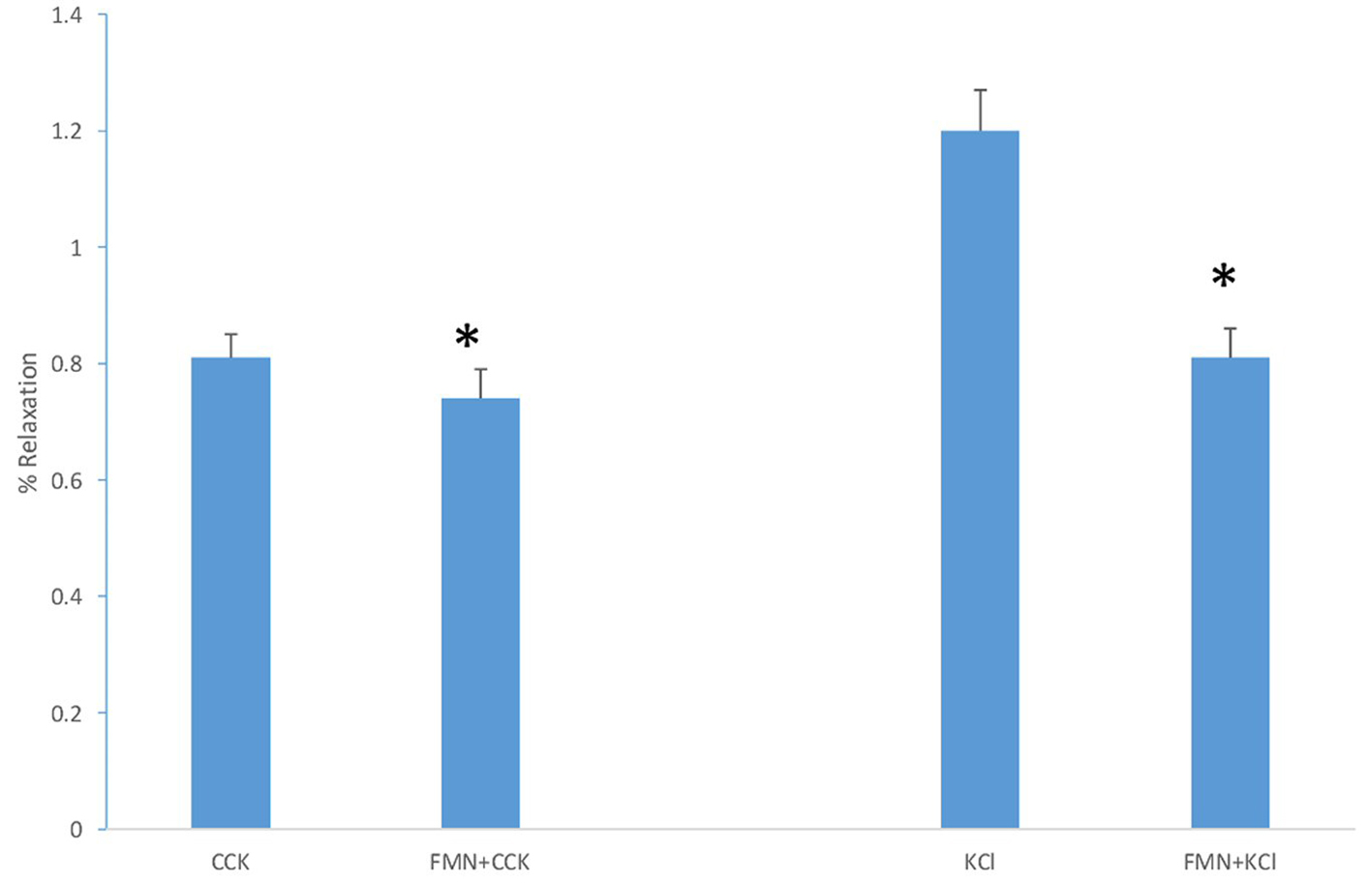 Click for large image | Figure 4. The effect of adding FMN (15 M) prior to KCl or CCK. The FMN significantly (P < 0.001, n = 9) decreased the amount of KCl-induced tension. A similar result (P < 0.01, n = 7) was observed when FMN was added to the chambers prior to the CCK. |
In order to determine if the effects of FMN and E2 were additive, strips taken from male guinea pigs were exposed to either FMN (15 M), E2 (50 M), or both as close together as possible. The amount of relaxation of 1.0 nM CCK-induced tension was recorded. The results can be seen in Table 1. When the amount of FMN-induced relaxation was compared with the amount of E2-induced relaxation, a significant difference was observed (FMN 51.4±2.7% vs. E2 61.1±2.5%). When the amount of FMN-induced relaxation of CCK-induced tension was compared with that from strips exposed to both FMN and E2 in combination, there was a significantly (P < 0.05, n = 28) more relaxation observed. Likewise, when the amount of E2-induced relaxation was compared with that obtained when both FMN and E2 were used in combination, there was significantly (P < 0.05) more relaxation than when E2 was used alone (Table 1).
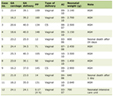 Click to view | Table 1. The Effect of FMN or E2 on the Relaxation of Male Guinea Pig Gallbladder Strips (FMN and E2 Separately and in Combination) |
FMN seemed to be more effective in relaxing CCK-induced tension than E2 as can be seen in Table 2. When a similar concentration of FMN and E2 was used (10 M), FMN induced significantly (P < 0.001) more relaxation of CCK-induced tension than the E2 (FMN 40.4±2.5% vs. E2 17.8±1.9%). FMN (20 M) and 100 M E2 (63.7±3.5%) induced similar amounts of relaxation of CCK-induced tension; however, in spite of a concentration of E2 5 times more than the FMN, there was no significant difference in the amount of relaxation induced (FMN 68.3±4.9% vs. E2 63.7±3.5%).
 Click to view | Table 2. Comparison of the Relaxation of CCK-Induced Tension Induced by Formononetin When Compared With E2 |
| Discussion | ▴Top |
Estrogens have an important hormonal role in all vertebrates. These hormones are required for normal gestation and embryonic development. Estrogens also regulate the fertility of adult females [18]. Extracts of red clover (Trifolium pratense) have been marketed as a natural product to relieve the hot flashes and other menopausal symptoms [19]. Commercial extracts of red clover contain high but varying amounts of estrogenic isoflavones, including FMN. These extracts have estrogenic potential to interact with ER and ER [20, 21].
FMN has been shown to affect the motility of vascular smooth muscle. Wu et al [8] using isolated rat aorta demonstrated that FMN relaxed aortic rings via endothelium-dependent and endothelium-independent pathways. The endothelium-dependent pathway utilized NO. The endothelium-independent pathway was mediated by BKCa and KATP pathways. Neither estrogen nor progesterone receptors were involved in the FMN effect. Zhao et al [9] also demonstrated that the relaxant effect of FMN was mediated, in part, by a NO-dependent pathway. The opening of K+ channels was also involved in the FMN-induced vasorelaxation. Sun et al [10] further confirmed the role of NO in mediating the FMN-induced relaxation of rat superior mesenteric arteries. In addition, they demonstrated that FMN upregulated eNOS (nitric oxide synthase) expression in the mesenteric arteries via estrogen receptors, ERK1.2, and JNK pathways. The finding that the effect of FMN is mediated through estrogen receptors differs from the results of Wu et al [8]. The use of L-NMMA in the guinea pig gallbladder strips had no significant effect on the amount of FMN-induced relaxation. Fulvestrant increased the amount of FMN-induced relaxation which suggested that fulvestrant was inhibiting other on-going activity which allowed the effects of FMN to be more prominent.
FMN was also shown to inhibit smooth muscle contraction of the isolated rat prostate gland; however, no mechanism of action was discussed [21].
In the guinea pig gallbladder the FMN-induced relaxation was also mediated by inhibiting protein tyrosine kinase. Wu et al [22] using the patch-clamp technique demonstrated that gallbladder contraction required increasing Ca2+ influx through L-type calcium channels via a PKC pathway. Since the FMN-induced relaxation was significantly decreased when PKC blockers were used or when FMN was added to the chambers prior to either CCK or KCl, it can be concluded that the FMN-relaxation is mediated by blocking extracellular Ca2+ entry and with a PKC-mediated pathway.
Phytoestrogens have the potential to be an alternative to estrogen therapy [7, 11]. E2 has been shown to relax CCK-induced tension in male guinea pig strips by inhibiting extracellular Ca2+ entry [16]. FMN-induced relaxation of CCK-induced tension was mediated, in part, by blocking extracellular Ca2+ just as E2 does. Both FMN and E2 seem to utilize some of the same intracellular signaling systems. Using FMN and E2 together it has been shown that the effects of FMN and E2 are additive, suggesting that both may be acting through similar pathways and that the amount of each agent used alone may not have activated all potential receptor sites. In addition, FMN appeared more potent in its effects on relaxing CCK-induced tension which may be important if FMN is used as an alternative to estrogen replacement therapy.
This is the first report that the isoflavone, FMN, has an effect on guinea pig gallbladder smooth muscle. FMN relaxed either CCK- or KCl-induced tension in a concentration dependent manner. The relaxant effect was mediated by FMN blocking extracellular Ca2+ entry, blocking the action of PKC, and blocking the action of protein tyrosine kinase. The relaxant action of FMN is mediated by multiple signaling pathways.
Financial Support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
| References | ▴Top |
- Yoshida Y, Wang MQ, Liu JN, Shan BE, Yamashita U. Immunomodulating activity of Chinese medicinal herbs and Oldenlandia diffusa in particular. Int J Immunopharmacol. 1997;19(7):359-370.
doi - Bensky D, Gamble A. Chinese Herbal Medicine: Materia Medica Revised Edition. Eastland Press, Seattle, 1993.
- Zhang S, Tang X, Tian J, Li C, Zhang G, Jiang W, Zhang Z. Cardioprotective effect of sulphonated formononetin on acute myocardial infarction in rats. Basic Clin Pharmacol Toxicol. 2011;108(6):390-395.
doi pubmed - Ombra MN, d'Acierno A, Nazzaro F, Riccardi R, Spigno P, Zaccardelli M, Pane C, et al. Phenolic composition and antioxidant and antiproliferative activities of the extracts of twelve common bean (Phaseolus vulgaris L.) endemic ecotypes of southern italy before and after cooking. Oxid Med Cell Longev. 2016;2016:1398298.
- Delgado-Zamarreno MM, Perez-Martin L, Bustamante-Rangel M, Carabias-Martinez R. A modified QuEChERS method as sample treatment before the determination of isoflavones in foods by ultra-performance liquid chromatography-triple quadrupole mass spectrometry. Talanta. 2012;100:320-328.
doi pubmed - Murkies AL, Wilcox G, Davis SR. Clinical review 92: Phytoestrogens. J Clin Endocrinol Metab. 1998;83(2):297-303.
pubmed - Dubey RK, Gillespie DG, Imthurn B, Rosselli M, Jackson EK, Keller PJ. Phytoestrogens inhibit growth and MAP kinase activity in human aortic smooth muscle cells. Hypertension. 1999;33(1 Pt 2):177-182.
doi pubmed - Wu JH, Li Q, Wu MY, Guo DJ, Chen HL, Chen SL, Seto SW, et al. Formononetin, an isoflavone, relaxes rat isolated aorta through endothelium-dependent and endothelium-independent pathways. J Nutr Biochem. 2010;21(7):613-620.
doi pubmed - Zhao Y, Chen BN, Wang SB, Wang SH, Du GH. Vasorelaxant effect of formononetin in the rat thoracic aorta and its mechanisms. J Asian Nat Prod Res. 2012;14(1):46-54.
doi pubmed - Sun T, Cao L, Ping NN, Wu Y, Liu DZ, Cao YX. Formononetin upregulates nitric oxide synthase in arterial endothelium through estrogen receptors and MAPK pathways. J Pharm Pharmacol. 2016;68(3):342-351.
doi pubmed - Beck V, Rohr U, Jungbauer A. Phytoestrogens derived from red clover: an alternative to estrogen replacement therapy? J Steroid Biochem Mol Biol. 2005;94(5):499-518.
doi pubmed - Kline LW, Kaneko T, Benishin CG, Pang PK. Calcitonin gene-related peptide: an inhibitor of guinea pig gallbladder contraction. Can J Physiol Pharmacol. 1991;69(8):1149-1154.
doi pubmed - Kline LW, Pang PK. Calcitonin gene related peptide relaxes cholecystokinin-induced contraction in guinea pig gallbladder strips in vitro. Can J Physiol Pharmacol. 1992;70(12):1571-1575.
doi - Kline LW, Karpinski E. 17beta-Estradiol relaxes cholecystokinin- and KCl-induced tension in male guinea pig gallbladder strips. Steroids. 2011;76(6):553-557.
doi pubmed - Kline LW, Karpinski E. Testosterone and dihydrotestosterone inhibit gallbladder motility through multiple signalling pathways. Steroids. 2008;73(11):1174-1180.
doi pubmed - Kline L, Karpinski E. A comparison of the effects of various sex steroids on cholecystokinin- and KCl-induced tension in female guinea pig gallbladder strips. Gen Comp Endocrinol. 2013;185:37-43.
doi pubmed - Kline LW, Karpinski E. Progesterone inhibits gallbladder motility through multiple signaling pathways. Steroids. 2005;70(9):673-679.
doi pubmed - Boron WF, Boulpaep EL. Medical Physiology, 3rd edition. Saunders Elsevier, Philadelphia, PA. 2017; p 1139-1142.
- Coon JT, Pittler MH, Ernst E. Trifolium pratense isoflavones in the treatment of menopausal hot flushes: a systematic review and meta-analysis. Phytomedicine. 2007;14(2-3):153-159.
doi pubmed - Andres S, Hansen U, Niemann B, Palavinskas R, Lampen A. Determination of the isoflavone composition and estrogenic activity of commercial dietary supplements based on soy or red clover. Food Funct. 2015;6(6):2017-2025.
doi pubmed - Brandli A, Simpson JS, Ventura S. Isoflavones isolated from red clover (Trifolium pratense) inhibit smooth muscle contraction of the isolated rat prostate gland. Phytomedicine. 2010;17(11):895-901.
doi pubmed - Wu ZX, Yu BP, Xia H, Xu L. Emodin increases Ca(2+) influx through L-type Ca(2+) channel in guinea pig gallbladder smooth muscle. Eur J Pharmacol. 2008;595(1-3):95-99.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cellular and Molecular Medicine Research is published by Elmer Press Inc.



