| Cellular and Molecular Medicine Research, ISSN 2817-6359 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cell Mol Med Res and Elmer Press Inc |
| Journal website https://www.thecmmr.org |
Review
Volume 1, Number 1, September 2023, pages 3-7
Advances in Molecular Perspectives of Tumor-Initiating Cells on Cancer Therapy
Licun Wu
Latner Thoracic Surgery Research Laboratories, Division of Thoracic Surgery, Toronto General Hospital, University Health Network, University of Toronto, Toronto, ON M5G 1L7, Canada
Manuscript submitted April 15, 2023, accepted June 26, 2023, published online August 30, 2023
Short title: Tumor-Initiating Cells
doi: https://doi.org/10.14740/cmmr10e
| Abstract | ▴Top |
Cancer stem cells (CSCs) known as tumorigenic cells are biologically distinct from diverse subpopulations. Cancer cell heterogeneity readily leads to development of drug resistance and tolerance to treatment. CSC hypothesis has resulted in incredible impact on the understanding and insight into tumor biology. More importantly, advances in molecular perspectives have achieved in the recent decades although many aspects of this hypothesis remain speculative and are still evolving. CSC has been considered a new cellular target for anticancer drug discovery. Along with identification of different CSC markers such as CD133, CD24, CD44, CD90 and signaling pathways such as Wnt/β-catenin, hedgehog and so on, different kinds of therapeutic approaches have been developed to work on these molecular targets, resulting in selective inhibition of CSC functions including self-renewal and differentiation. Most recent studies demonstrated that CR1 expression in colon CSC can promote the stem cell clone formation, CCR7 promotes breast CSC growth, TP53 splice can enhance the pluripotency of CSC through the positive regulation of Sox2, Oct3/4 and Nanog and other key factors, to increase the potential risk of cancer recurrence, and an exciting finding is that carbon nanomaterials may be used as a CSC sniper. Selectively targeting CSC has shown promising perspectives and may open a new venue for the treatment of cancer.
Keywords: Tumor initiating cell; Cancer stem cell; Biomarker; Molecular target; Cancer therapy
| Introduction | ▴Top |
Tumor-initiating cells (TICs), also known as cancer stem cells (CSCs), are tumorigenic cells that are biologically distinct from diverse subpopulations [1, 2]. Cancer cell heterogeneity readily leads to development of drug resistance and tolerance to treatment [3, 4]. CSC hypothesis has resulted in incredible impact on the understanding and insight into tumor biology. CSCs have been identified in a wide variety of human tumors in the recent decades [5-9]. Within heterogeneous cancer cell population of the tumors, CSCs are tumorigenic cells and are biologically distinct from other subpopulations. CSCs are characterized by self-renewal and differentiation which drive tumor progression [5, 10-12].
The hypothesis of CSC includes stochastic model and hierarchy model. Stochastic cell model proposed that each single tumor cell is tumorigenic. All tumor cells are equipotent and can self-renew or differentiate so as to maintain the property of tumor heterogeneity, resulting in diverse cells in phenotypes within a tumor [13, 14].
While the concept of CSC model (or hierarchy cell model) proposes that a tumor is a heterogeneous population of mutant cells. A tumor may have various phenotypes of CSC. Certain type of CSC plays critical role in maintaining successful adaptation to tumor environment, which makes it possible to develop CSC-specific treatment regimens by therapeutic intervention [15, 16].
Tumor hierarchy model has been considered as a fundamental concept in tumor biology and promises a new cellular target for anticancer drug discovery. Although the CSC hypothesis was first proposed decades ago, many aspects of this hypothesis remain speculative and are still evolving [17, 18].
The clonal evolution model, which occurs in both stochastic model and hierarchy model, postulates that mutant tumor cells with a growth advantage outproliferate others. Cells in the dominant population have a similar potential for initiating tumor growth. Dick et al proposed that the genetics and CSC models can be harmonized by genetic diversity and non-genetic influences in contributing to tumor heterogeneity. Therefore, a better interpretation of previous observation may be offered through integrating CSC and cancer genetics [16].
CSCs may be the cause of drug resistance and tolerance to treatment
Considerable evidence has indicated that CSCs have a strong ability to transform thus often escape the killing of therapies, which is now the major problem in the field of cancer treatment.
Development of drug resistance limits the efficacy of treatment, partly due to cancer cell heterogeneity. Evidence indicates that CSCs are usually more resistant to the conventional therapies leading to clinical relapse [19].
CD133, a putative stem cell marker in malignant brain tumors, enhances multidrug resistant gene 1 (MDR1) expression following chemotherapy in adult malignant glioblastomas. In this study they found that CD133 and MDR1 were co-expressed and their expression was elevated in recurrent glioblastoma from patients who received chemotherapy. PI3K-Akt-NF-κB signaling mediator expression was also elevated in the chemotherapy-resistant patients. Suppressing CD133 expression decreased levels of PI3K-Akt-NF-κB and MDR1, but improved chemosensitivity [20].
Secreted Wnt signals are associated with maintenance of stem cell property. Mouse and human lung adenocarcinomas display hierarchical features with two distinct subpopulations either with high or low Wnt signaling activity. The Wnt responder cells showed increased tumor propagation ability with CSC features. Wnt inhibitors reduced tumor growth and markedly decreased the proliferative potential of lung cancer cells in mice, indicating that strategies for disrupting pathways that maintain CSC phenotypes can translate into effective anti-cancer therapies [21].
For taxanes, a mainstay of treatment for breast cancer, drug resistance easily occurs. Breast cancer patient-derived xenografts were used to study the underlying mechanisms. They identified a CD49f+ chemoresistant population with tumor-initiating ability which expands during the acquisition of drug resistance. The resistant CD49f+ population shrinks and taxane sensitivity can be restored in the absence of drug treatment. CD49f+ enriched cells were chemoresistant with TIC properties, revealing the potential mechanism of acquired resistance to docetaxel in triple-negative breast cancer [22].
Based on the current studies, since CSC can not differentiate into other special cell types, it would be feasible to develop new strategies to selectively kill CSC, thereby inhibiting the development of cancer. More importantly, inhibition of CSC would be able to improve efficacy of treatment by enhancing the sensitivity to chemotherapeutic drugs.
Promising candidate biomarkers to identify CSCs
Nowadays it has been widely accepted that CSC may be resistant to conventional cancer therapies, especially chemotherapy and radiotherapy [23]. Much effort is being made to identify therapeutic strategies that could target CSC. This review attempts to summarize recent advances in the CSC research, casting a light of hope on the potential approaches for anticancer drug development (Table 1) [8, 24-38]. Based on the above CSC biomarkers or pathways, novel approaches targeting CSC have been developed (Fig. 1). Clinical trial “The immunotherapy of nasopharyngeal cancer using cancer stem cell vaccine (NCT02115958)” was sponsored by Fuda Cancer Hospital, Guangzhou, China and collaborated with University of Michigan, MI, USA. They examined the vaccination effects produced by enriched CSC and found that CSC vaccination was immunogenic and more effective as an antigen source than bulk tumor cells in inducing antitumor immunity (https://clinicaltrials.gov).
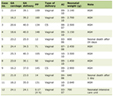 Click to view | Table 1. A Few Examples of Molecules Are Used as Potential Markers to Identify Cancer Stem Cells |
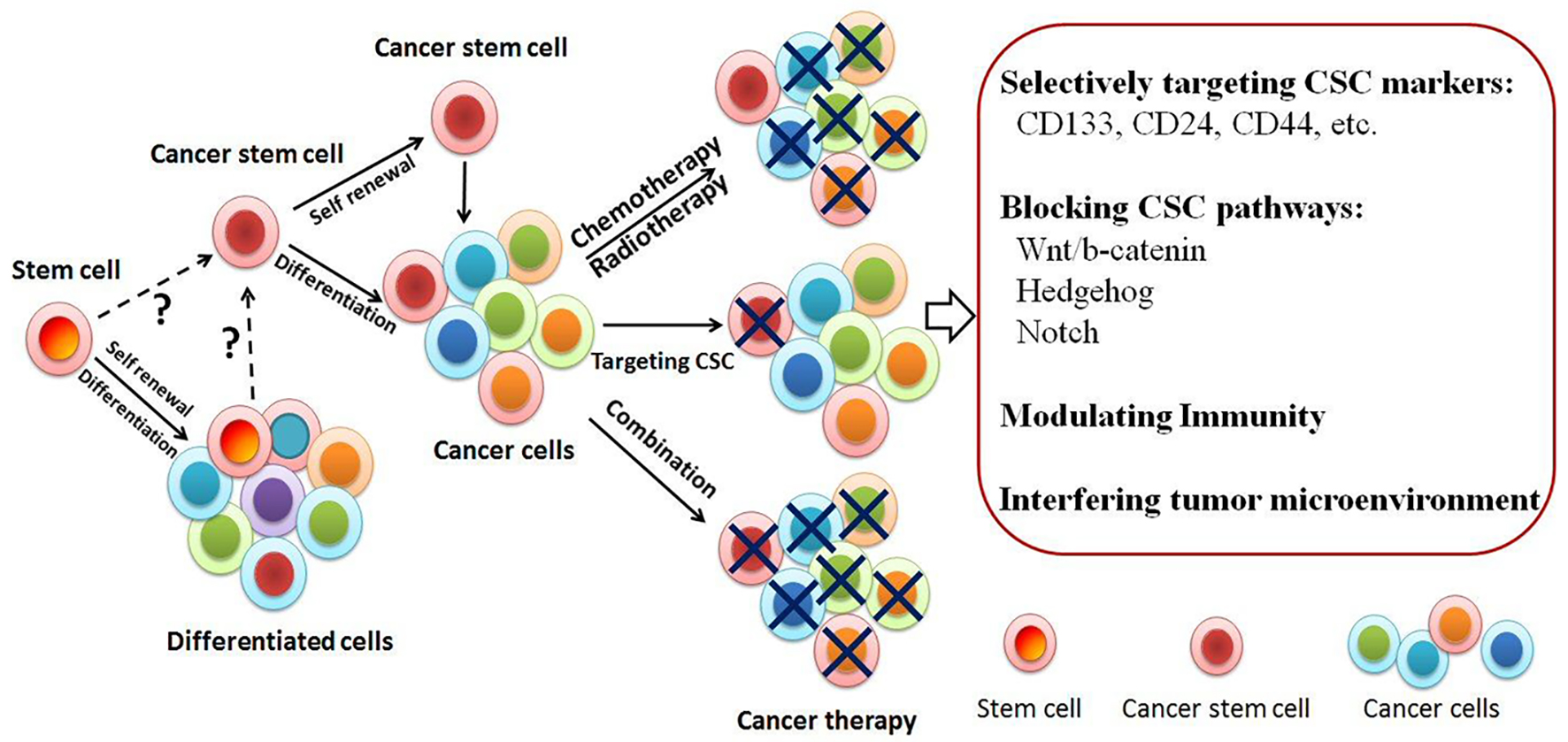 Click for large image | Figure 1. Tumor initiation models. Tumor-initiating cells/cancer stem cells (TICs/CSCs) may be the critical target to eradicate tumor. The origin of CSC is unknown yet. It might come from stem cells or somatic cells. CSCs known as tumorigenic cells are biologically distinct from diverse subpopulations. Cancer cell heterogeneity often leads to resistance to chemoradiation treatment. Drug-resistant cells contain CSC enrichment. Novel approaches targeting CSC have been developed either targeting the surface molecules or blocking the pathways. Selectively targeting CSC has shown promising and may open a new venue for the treatment of cancer. |
| New Emerging Therapeutic Approaches to Targeting CSC | ▴Top |
The most recent studies demonstrated that CR1 expression in colon CSC can promote the stem cell clone formation, CCR7 promotes breast CSC growth, TP53 splice can enhance the pluripotency of CSC through the positive regulation of Sox2, Oct3/4 and Nanog and other key factors, to increase the potential risk of cancer recurrence, and an exciting finding is that carbon nanomaterials may be used as a CSC sniper [39-42]. Current achievements indicate that CSC may open a promising venue for the treatment of cancer.
Cripto-1 regulates colon CSC function
Recently, a new study from Italian group found that embryonic protein Cripto-1 (CR1) expression in colon CSC can promote the stem cell clone formation, showing that CR1 may promote colon cancer recurrence by promoting CSC growth [39].
Stemness is a dynamic change that may be present in both normal and tumor cells. Embryonic protein CR1 can be expressed in normal stem cells at the bottom of the colon’s crypts and also in CSC of colon cancer tissues. CR1-positive cell subsets were found by sorting the tumor tissue of colon cancer patients, and these cells had stronger clonal ability to express stem cell-related genes at the same time.
CR1 expression in tumor cells may change over time and is regulated by intracellular proteins, cell surface proteins and secretory proteins, and these regulatory correlations are associated with the clonal formation ability of CR1-positive subgroups. Inhibition of CR1 expression in vitro was able to induce CSC growth. This inhibition was accompanied by a down-regulation of the Src/Akt signaling pathway, and it was also demonstrated by in vivo experiments that silencing CR1 inhibited CSC-driven tumor growth and reduced the number of CSC. The use of an inducible expression system to silence CR1 in established tumor implants can inhibit the growth of CSC, and this inhibitory effect is present in both primary and metastatic tumors, demonstrating that CR1 is important for CSC growth. These results suggest that CR1 is a novel dynamic factor for regulating the function of colon CSC, and may become an important target for the treatment of colon cancer, inhibiting CSC function and preventing cancer recurrence.
Chemokine receptor CCR7 promotes breast CSC growth
An Australia group recently reported their latest findings that chemokine receptor CCR7 regulates the growth of CSC in breast cancer, suggesting that CCR7 may be a potential target for cancer treatment [40].
CCR7 is widely detected in breast cancer pathology. Although recent studies have shown that high levels of CCR7 expression are associated with advanced tumor grade and poor prognosis, in vivo studies on their specific function in breast cancer and the molecular mechanisms involved in breast cancer are still very limited.
To address these issues, they used CCR7-deficient breast cancer mouse model and found that CCR7 deletion resulted in a significant lag in breast cancer and a significant decrease in tumor burden. Through mechanism studies, it was found that in human and mouse tumor cells, CCR7 can function by regulating the stemness of CSC. In vivo experiments showed that inhibition of CCR7 activity by gene deletion or drug blockade can significantly reduce the number of primary breast cancer cells in mice, which provides a reasonable mechanism for CCR7 to promote tumor growth.
These results revealed that the oncogene properties of CCR7 in mammary epithelial tumors provide a potential target for the development of therapeutic intervention for targeting CSC.
P53 subtype promotes CSC potential
A new study of French scientists recently found that a TP53 splice can enhance the pluripotency of CSC through the positive regulation of Sox2, Oct3/4 and Nanog and other key factors, to increase the potential risk of cancer recurrence [41, 43].
In this study, they found that a TP53 splice could enhance the stemness in breast cancer cells MCF-7 and reduce the stemness after deletion of such splice. This TP53 splice can stimulate the expression of the pluripotent factors Sox2, Oct3/4 and Nanog. At the same time, in other highly metastatic breast cancer cells, invasive and CSC potential enhancement and TP53 splice expression increased, and the expression of Sox2, Oct3/4 and Nanog is also subject to positive regulation of TP53 splice. The use of anti-tumor drug etoposide to treat MCF-7 cells can promote the CSC formation and enhance the expression of Sox2, Oct3/4 and Nanog in TP53 splice-dependent, increasing the potential risk of cancer recurrence.
TP53 was used to be thought mainly as a tumor suppressor; however, this study shows that a splice of TP53 can promote the potential of CSC, suggesting that TP53 splice may also play a role of oncogene.
Nanoparticles target and kill CSCs that drive tumor growth
A Chinese group found that metallofullerenol nanomaterial Gd@C82(OH)22 can be used as a drug delivery agent, and effectively inhibit the self-renewal ability of CSC in breast cancer with triple-negative biomarkers. This nanoparticle may block epithelial-to-mesenchymal transition (EMT) through regulating tumor microenvironment to achieve efficient removal of CSC, thereby preventing tumor initiation and metastasis.
CSC is the leading cause of cancer recurrence and metastasis, because CSC is more resistant to chemotherapy and radiotherapy. Identifying new drugs that could effectively target “sniper” CSC will be expected to become a new hope for cancer.
In this study, nanoparticles were used in the tumor surface of the oxygen-rich microenvironment (rich tumor neovascularization) and deep tumor hypoxic microenvironment through transition from deprotonation to protonation, to achieve more effective breast cancer treatment by targeting the “sniper” CSC.
High toxicity of CSC drugs found so far is a key issue that limits its clinical application. In contrast, in vitro and in vivo experiments have shown that such carbon nanomaterials have no observable toxicity. It thus becomes a non-toxic nanocomposite that can directly target CSC.
CSC themselves are highly heterogeneous, and similar with tumor cells. Current anti-CSC drugs often can partially attenuate at individual targets rather than effectively kill CSC, but also increase the toxicity to normal stem cells. Due to the unique physicochemical properties of the fullerene nanostructures, especially the high degree of controllability of the surface of the sphere structure, it is non-toxic to normal stem cells. There are several systemic reviews that were recently published, specifically on targeting CSC by using the nanoparticles [44, 45]. Therefore, it would be more practical for the clinical application [42].
Concluding Remarks
CSC theory provides a novel direction and perspective to understand tumor origin, the diagnosis, and new drug development for cancer therapy. Recently, the subject of CSC is frequently published in the high impact journals, showing its promising value of clinical application. Development of drugs that are selectively targeting CSC would be a potential approach to the treatment of cancer patients.
Conflicts of Interest
There are no financial conflicts of interest.
| References | ▴Top |
- Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, Weinberg RA. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525(7568):256-260.
doi pubmed pmc - Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324(5935):1670-1673.
doi pubmed pmc - Bao B, Mitrea C, Wijesinghe P, Marchetti L, Girsch E, Farr RL, Boerner JL, et al. Treating triple negative breast cancer cells with erlotinib plus a select antioxidant overcomes drug resistance by targeting cancer cell heterogeneity. Sci Rep. 2017;7:44125.
doi pubmed pmc - Oshimori N, Oristian D, Fuchs E. TGF-beta promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell. 2015;160(5):963-976.
doi pubmed pmc - Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730-737.
doi pubmed - Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396-401.
doi pubmed - O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106-110.
doi pubmed - Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13(2):153-166.
doi pubmed - Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, et al. Identification of cells initiating human melanomas. Nature. 2008;451(7176):345-349.
doi pubmed pmc - Sullivan JP, Minna JD, Shay JW. Evidence for self-renewing lung cancer stem cells and their implications in tumor initiation, progression, and targeted therapy. Cancer Metastasis Rev. 2010;29(1):61-72.
doi pubmed pmc - Tysnes BB. Tumor-initiating and -propagating cells: cells that we would like to identify and control. Neoplasia. 2010;12(7):506-515.
doi pubmed pmc - Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23(43):7274-7282.
doi pubmed - Odoux C, Fohrer H, Hoppo T, Guzik L, Stolz DB, Lewis DW, Gollin SM, et al. A stochastic model for cancer stem cell origin in metastatic colon cancer. Cancer Res. 2008;68(17):6932-6941.
doi pubmed pmc - Schinazi RB. A stochastic model for cancer risk. Genetics. 2006;174(1):545-547.
doi pubmed - Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138(5):822-829.
doi pubmed - Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14(3):275-291.
doi pubmed - Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105-111.
doi pubmed - Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3(12):895-902.
doi pubmed - Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017.
doi pubmed pmc - Xi G, Li YD, Grahovac G, Rajaram V, Wadhwani N, Pundy T, Mania-Farnell B, et al. Targeting CD133 improves chemotherapeutic efficacy of recurrent pediatric pilocytic astrocytoma following prolonged chemotherapy. Mol Cancer. 2017;16(1):21.
doi pubmed pmc - Tammela T, Sanchez-Rivera FJ, Cetinbas NM, Wu K, Joshi NS, Helenius K, Park Y, et al. A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature. 2017;545(7654):355-359.
doi pubmed - Gomez-Miragaya J, Palafox M, Pare L, Yoldi G, Ferrer I, Vila S, Galvan P, et al. Resistance to Taxanes in Triple-Negative Breast Cancer Associates with the Dynamics of a CD49f+ Tumor-Initiating Population. Stem Cell Reports. 2017;8(5):1392-1407.
doi pubmed pmc - Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8(10):806-823.
doi pubmed - Toren A, Bielorai B, Jacob-Hirsch J, Fisher T, Kreiser D, Moran O, Zeligson S, et al. CD133-positive hematopoietic stem cell "stemness" genes contain many genes mutated or abnormally expressed in leukemia. Stem Cells. 2005;23(8):1142-1153.
doi pubmed - Sansone P, Ceccarelli C, Berishaj M, Chang Q, Rajasekhar VK, Perna F, Bowman RL, et al. Self-renewal of CD133(hi) cells by IL6/Notch3 signalling regulates endocrine resistance in metastatic breast cancer. Nat Commun. 2016;7:10442.
doi pubmed pmc - Zimmerer RM, Ludwig N, Kampmann A, Bittermann G, Spalthoff S, Jungheim M, Gellrich NC, et al. CD24+ tumor-initiating cells from oral squamous cell carcinoma induce initial angiogenesis in vivo. Microvasc Res. 2017;112:101-108.
doi pubmed - Jafari SM, Joshaghani HR, Panjehpour M, Aghaei M, Zargar Balajam N. Apoptosis and cell cycle regulatory effects of adenosine by modulation of GLI-1 and ERK1/2 pathways in CD44+ and CD24- breast cancer stem cells. Cell Prolif. 2017.
doi pubmed - Panaccione A, Zhang Y, Ryan M, Moskaluk CA, Anderson KS, Yarbrough WG, Ivanov SV. MYB fusions and CD markers as tools for authentication and purification of cancer stem cells from salivary adenoid cystic carcinoma. Stem Cell Res. 2017;21:160-166.
doi pubmed - Roudi R, Madjd Z, Korourian A, Mehrazma M, Molanae S, Sabet MN, Shariftabrizi A. Clinical significance of putative cancer stem cell marker CD44 in different histological subtypes of lung cancer. Cancer Biomark. 2014;14(6):457-467.
doi pubmed - Yan X, Luo H, Zhou X, Zhu B, Wang Y, Bian X. Identification of CD90 as a marker for lung cancer stem cells in A549 and H446 cell lines. Oncol Rep. 2013;30(6):2733-2740.
pubmed - Miyazaki Y, Matsubara S, Ding Q, Tsukasa K, Yoshimitsu M, Kosai K, Takao S. Efficient elimination of pancreatic cancer stem cells by hedgehog/GLI inhibitor GANT61 in combination with mTOR inhibition. Mol Cancer. 2016;15(1):49.
doi pubmed pmc - Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422(6929):313-317.
doi pubmed - Li J, Yu B, Deng P, Cheng Y, Yu Y, Kevork K, Ramadoss S, et al. KDM3 epigenetically controls tumorigenic potentials of human colorectal cancer stem cells through Wnt/beta-catenin signalling. Nat Commun. 2017;8:15146.
doi pubmed pmc - Zhang K, Guo Y, Wang X, Zhao H, Ji Z, Cheng C, Li L, et al. WNT/beta-Catenin Directs Self-Renewal Symmetric Cell Division of hTERThigh Prostate Cancer Stem Cells. Cancer Res. 2017;77(9):2534-2547.
doi pubmed - Pannuti A, Foreman K, Rizzo P, Osipo C, Golde T, Osborne B, Miele L. Targeting Notch to target cancer stem cells. Clin Cancer Res. 2010;16(12):3141-3152.
doi pubmed pmc - Lagadec C, Vlashi E, Alhiyari Y, Phillips TM, Bochkur Dratver M, Pajonk F. Radiation-induced Notch signaling in breast cancer stem cells. Int J Radiat Oncol Biol Phys. 2013;87(3):609-618.
doi pubmed pmc - Cordone I, Masi S, Summa V, Carosi M, Vidiri A, Fabi A, Pasquale A, et al. Overexpression of syndecan-1, MUC-1, and putative stem cell markers in breast cancer leptomeningeal metastasis: a cerebrospinal fluid flow cytometry study. Breast Cancer Res. 2017;19(1):46.
doi pubmed pmc - Alam M, Ahmad R, Rajabi H, Kharbanda A, Kufe D. MUC1-C oncoprotein activates ERK-->C/EBPbeta signaling and induction of aldehyde dehydrogenase 1A1 in breast cancer cells. J Biol Chem. 2013;288(43):30892-30903.
doi pubmed pmc - Francescangeli F, Contavalli P, De Angelis ML, Baiocchi M, Gambara G, Pagliuca A, Fiorenzano A, et al. Dynamic regulation of the cancer stem cell compartment by Cripto-1 in colorectal cancer. Cell Death Differ. 2015;22(10):1700-1713.
doi pubmed pmc - Boyle ST, Ingman WV, Poltavets V, Faulkner JW, Whitfield RJ, McColl SR, Kochetkova M. The chemokine receptor CCR7 promotes mammary tumorigenesis through amplification of stem-like cells. Oncogene. 2016;35(1):105-115.
doi pubmed - Kramer AC, Weber J, Zhang Y, Tolar J, Gibbens YY, Shevik M, Lund TC. TP53 Modulates Oxidative Stress in Gata1+ Erythroid Cells. Stem Cell Reports. 2017;8(2):360-372.
doi pubmed pmc - Liu Y, Chen C, Qian P, Lu X, Sun B, Zhang X, Wang L, et al. Gd-metallofullerenol nanomaterial as non-toxic breast cancer stem cell-specific inhibitor. Nat Commun. 2015;6:5988.
doi pubmed pmc - Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663-676.
doi pubmed - Hong IS, Jang GB, Lee HY, Nam JS. Targeting cancer stem cells by using the nanoparticles. Int J Nanomedicine. 2015;10(Spec Iss):251-260.
doi pubmed pmc - Qin W, Huang G, Chen Z, Zhang Y. Nanomaterials in Targeting Cancer Stem Cells for Cancer Therapy. Front Pharmacol. 2017;8:1.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cellular and Molecular Medicine Research is published by Elmer Press Inc.



